How to Close Out Sugaring Season on a Sweet Note
Leave a CommentAs the days grow warmer and trees start to bud, maple sugaring season winds down. The steady flow of sap slows as nighttime temperatures stay above freezing, and the changing trees trigger a chemical shift that can turn the sap bitter. When this happens, it’s time for syrup producers to wrap up operations and start planning for next year.
A successful sugaring season starts with proper end-of-season maintenance. Here’s how to ensure your maple syrup production is in top shape when the next season rolls around.
1. Inspect and Maintain Your Equipment
Maple syrup production relies on specialized equipment, from spiles and buckets to evaporators and heat exchangers. Before packing everything away, take time to inspect each piece. Check for cracks, warping, rust, or any other damage that could affect performance next year.
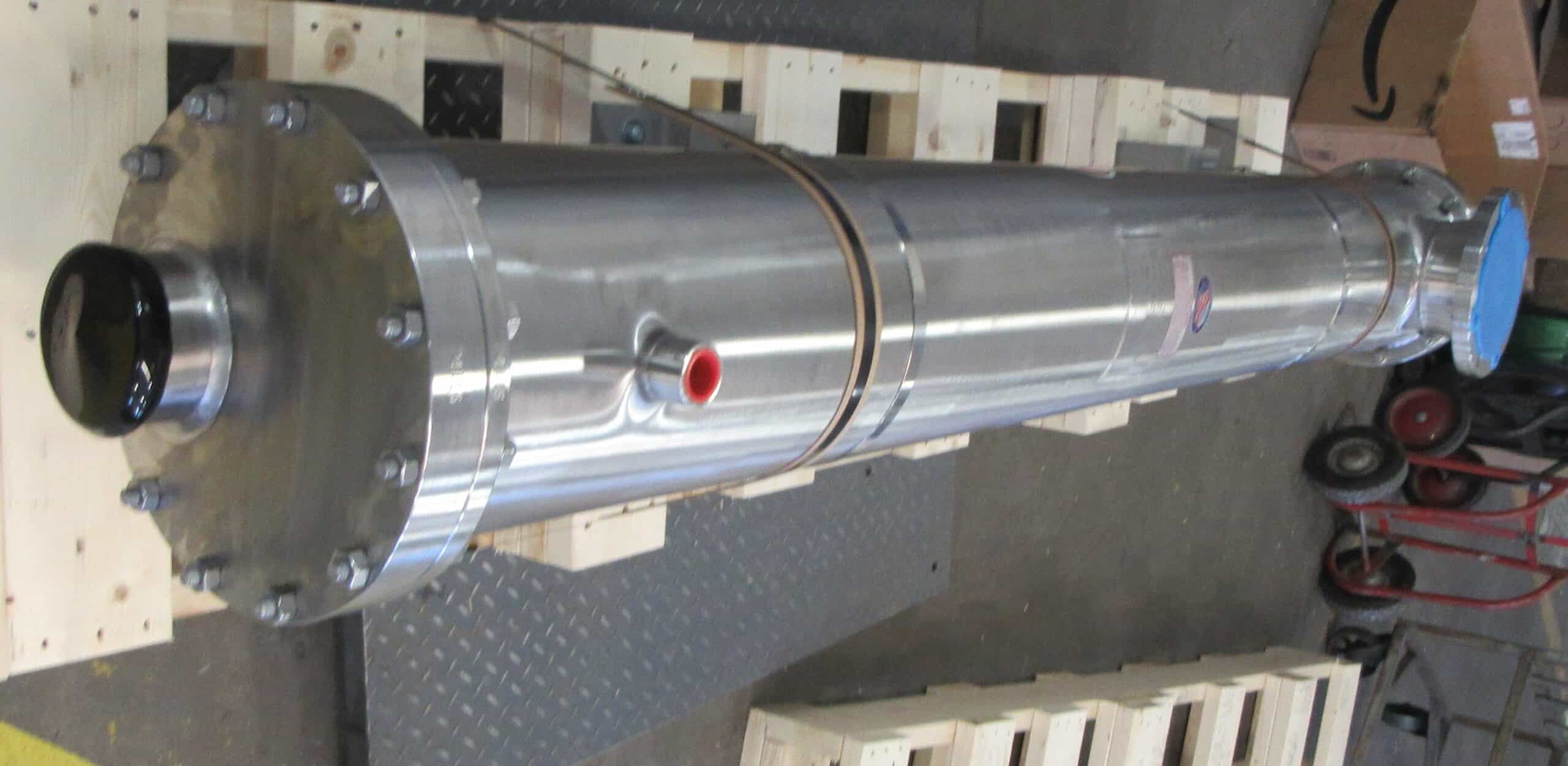
Pay special attention to shell and tube heat exchangers, which play a critical role in achieving the perfect syrup consistency and ensuring food safety. If anything needs replacing, now is the time—waiting until next season could leave you scrambling.
2. Evaluate Your Tappable Trees
High-quality syrup starts with healthy trees. Walk through your sugar bush to assess your maple trees, noting any that are dead, diseased, or damaged. Trees infested with insects or those producing lower-quality sap should be retired. Removing weaker trees allows healthier ones to thrive, ultimately improving your yield.
3. Clean Up for a Fresh Start
Maple syrup production is naturally sticky business. Sap, sugar residue, and outdoor debris can accumulate on equipment throughout the season. To keep everything in peak condition, thoroughly clean all tools, tanks, and tubing.
Use hot water as your primary cleaning agent—soaps and detergents can leave behind unwanted flavors that may taint next year’s syrup. For deeper sanitation, refer to manufacturer recommendations for safe and effective cleaning solutions.
Looking Ahead
While it’s always a little bittersweet to see the season end, it’s also the start of another important phase—bottling, selling, and enjoying the fruits of your labor. Proper maintenance now ensures a smoother, more productive sugaring season next year.
If you’re in the market for new equipment or need guidance on maintaining your shell and tube heat exchangers, the heat transfer experts at Enerquip are here to help. Get in touch to keep your operation running efficiently for seasons to come.
More from the Enerquip Blog
Maple Syrup Producers Use Heat Exchangers to Improve Production
Tube Side or Shell Side: Comparing Fluid Allocation Options for Your Shell and Tube Heat Exchanger
How Static Mixers & Turbulators Improve Heat Exchanger Efficiency
Honey Warming Prevents Crystallization
How Shell and Tube Heat Exchangers Benefit the Agriculture Industry